上海金畔生物科技有限公司代理Takara Clontech酶试剂盒全线产品,欢迎访问官网了解更多产品信息和订购。
| 人iPS细胞来源肝脏细胞 | ||||||
| 品牌 | Code No. | 产品名称 | 包装量 | 价格(元) | 说明书 | 数量 |
| Cellartis | Y10133 | Cellartis® Enhanced hiPS-HEP v2 (from ChiPSC12) Kit | 1 kit | ¥21,173 | |
  |
| Cellartis | Y10134 | Cellartis® Enhanced hiPS-HEP v2 (from ChiPSC18) Kit | 1 kit | ¥21,173 | |
  |
| Cellartis | Y10135 | Cellartis® Enhanced hiPS-HEP v2 (from ChiPSC22) Kit | 1 kit | ¥21,173 | |
  |
收藏产品 加入购物车
| 人iPS干细胞分化来的肝细胞 |
||||||||||
|
||||||||||
| ■ 产品详情请点击: |
||||||||||
 |
||||||||||
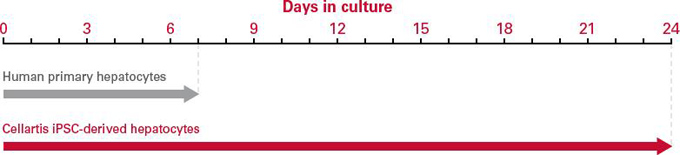
| Cellartis 人iPS细胞来源的肝脏细胞可以作为长期的、可靠的肝脏疾病模型。 | ||||||||||
| Cellartis human induced pluripotent stem (iPS) cell-derived hepatocytes are a long-lasting, reliable liver disease model. Ideal for long-term studies, these mature, functional, pure hepatocytes allow you to generate consistent results with low batch-to-batch variability. Cellartis iPSC-derived hepatocytes can be used for extended culture significantly longer than human primary hepatocytes—allowing you to get more data from your chronic toxicity studies. | ||||||||||
 |
||||||||||
| Cellartis enhanced hiPS-HEP cells可以稳定表达CYP450 活性超过21天。 | ||||||||||
| CYP450 activity is stable in enhanced hiPS-HEP cells over 21 days. LC/MS was used to analyze CYP450 activity in cultured enhanced hiPS-HEP cells previously derived from the hiPS cell lines ChiPSC12, ChiPSC18, and ChiPSC22 (abbreviated as C12, C18, and C22). CYP3A (Panel A), CYP2C9 (Panel B), CYP1A (Panel C), and CYP2C19 (Panel D) activities in enhanced hiPS-HEP cells are stable over an extended culture time. Cryopreserved human primary hepatocytes (hphep), which are functional in culture for a significantly shorter time than enhanced hiPS-HEP cells, were thawed and cultured for 20 hours, and their data are shown as the black bar in each panel. | ||||||||||
 |
||||||||||
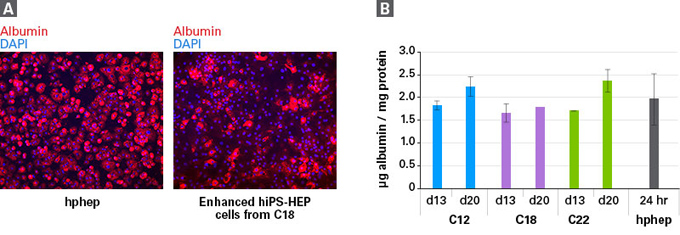
| Cellartis enhanced hiPS-HEP细胞在20天的培养中表达白蛋白(Albumin)。 | ||||||||||
| Albumin is present in enhanced hiPS-HEP cells through 20 days in culture. Panel A. Representative images of enhanced hiPS-HEP cells from human iPS cell line ChiPSC18 (C18) taken 12 days after thawing (right), compared to cryopreserved human primary hepatocytes (hphep) taken 24 hr after thawing (left). Cells were stained for albumin and DAPI. Panel B. Albumin secretion as measured by ELISA; n=2 for enhanced hiPS-HEP cells, with the exception of C18 at 20 days (n=1), and n=3 donors for hphep. | ||||||||||
| (以上图片均来源于Takara Bio USA, Inc.) | ||||||||||
| 参考文献: | ||||||||||
| 1. Heins et al. Stem Cells 2004; 22: 367-376.United States National Stem Cell Bank; http://www.nationalstemcellbank.org. 2. Mantel N et al. Potential markers of attenuation of YF virus after infection of stem cell-derived human hepatocytes with wild-type Asibi or live-attenuated YF17D virus.Supplement to The American Journal of tropical Medicine and Hygiene, Volume 83, November 2010, Number 5, abstract 12. 3. Yildirimman R et al. Human embryonic stem cell derived hepatocyte-like cells as a tool for in vitro hazard assessment of chemical carcinogenicity. Toxicol. Sci. 2011 Dec; 124(2): 278-90. 4. Ulvestad M et al. Drug metabolizing enzyme and transporter protein profi les of hepatocytes derived from human embryonic and induced pluripotent stem cells. Biochem Pharmacol. 2013 Sep 1; 86(5):691-702. |
||||||||||
