Laminarinase/Lichenase from Rhodothermus marinus
货号:P-BGLU110
规格:EA 200 Units
品牌: Jinpan
报价:¥3380.00
商品描述
A highly thermostable Laminarinase (Beta-glucanase). The enzyme is active on β-1-3 linkages in polysaccharides of D-glucose units with β-1-3 linkages including β-glucans with mixed linkages such as β-1-3 and β-1-4 or β-1-3 and β-1-6 linkages includinglaminarin, lichenan, barley beta-glucan and scleroglucan. Uniquely active on recalcitrant beta-glucans such as scleroglucanand curdlan.
The enzyme is available for purchase as 200 units size (see unit definition below).
Enzyme activity: ThermoActive™ Laminarinase catalyses thehydrolysis of β-1-3 linkages in polysaccharides of D-glucose residues connected by β-1-3 linkages or mixture of β-1-3 linkages and either β-1-4 linkages or β-1-6 linkages . This includes barley beta-glucan, laminarin, lichenin, scleroglucan and curdlan. The activity of the enzyme on barley β-glucan indicates that the enzyme is also able to hydrolyse β-1-4 linkages. The enzyme does not appear to hydrolyze β1-6 linkages and the extent of hydrolysis of Laminarin is dependent on the source of the substrate, i.e. proportion of β1-3 vs β1-6 linkages.
Activity determination: The standard assay for activity was done by incubating the enzyme at 75°C for 15 min. with 1% (w/v) lichenan as substrate in 0,1 M sodium phosphate buffer at pH 7.0. The reducing sugars released were detected by the dinitrosalisylic acid method using glucose as standard.
Unit definition: One unit (U) of enzyme activity is the amount that leads to the release of 1 μmol reducing sugars per minute.
Synonyms: glycoside hydrolase, 1,3-[1,3;1,4]-β-D-Glucan-3(4)-glucanohydrolase, Endo-1,3(4)-β-glucanase,laminaranase, lichenase, licheninase.
Protein family: Glycosyl hydrolase family 16 ( GH16 ) – CAZy database
Enzyme classification: EC 3.2.1.6
Source: The thermophilic bacteria Rhodothermus marinus
Protein sequence: NBCI protein entry
Structural information: The crystal structure of Laminarinase from Rhodothermus marinus (96% sequence identity with Bglu110 Laminarinase/Lichenase) has been determined to 1.9 Å resolution. – PDB entry 3ILN
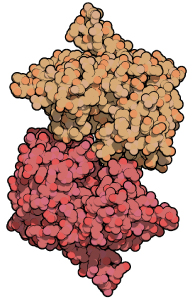
Crystal structure of Laminarinase from Rhodothermus marinus
Substrates:
The molecule laminarin (also known as laminaran) is a storage glucan produced in brown algae through photosynthesis. The polysaccharide is made up of glucose residues withβ-1,3-linkages and β-1,6-linkages. It is a linear polysaccharide, with a β(1→3):β(1→6) ratio typically 3:1 but the ratio may vary with the source of the polysaccharide.
bglu110-substrate

β-1-3 and β-1-6 linkages found in laminarin
Lichenin, also known as lichenan or moss starch, is a complex glucan occurring in certain species of lichensandconsists of repeating glucose units linked by β-1,3 and β-1,4 glycosidic bonds. It can be extracted from Cetraria islandica(Iceland moss).
Scleroglucan, is formed by the fungus Sclerotium rolfsii. A chemically analogous polysaccharide, Schizophyllan (Sizofiran, Sonifilan, SPG) is a neutral extracellular polysaccharide produced by the fungus Schizophyllum commune. Schizophyllan is a β-1,3 beta-glucan with β-1,6 branching. Both polysaccharides share the chemical structure of the backbone with curdlan.
Properties of Bglu110 Laminarinase/Lichenase (β-glucanase):
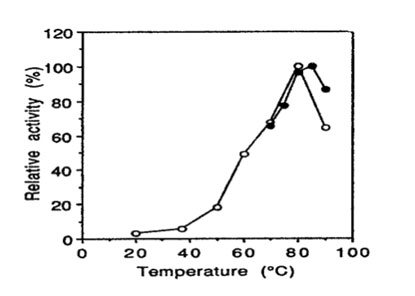
Temperature optimum: the enzyme has optimum activity around 80°C
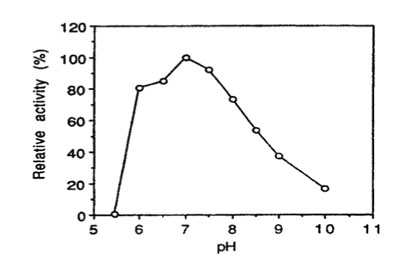
Bglu110 Beta-Glucanase activity vs. pH
pH optimum: the enzyme has optimum activity around pH 7
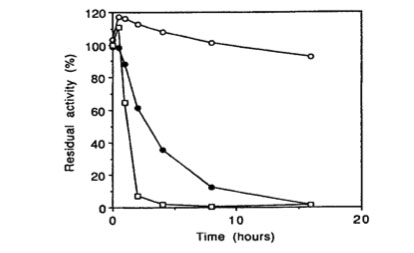
Bglu110 Beta-Glucanase thermostability
Thermostability: Activity of the enzyme as a function of time at different temperature
– ( open circles: 80°C; closed circles: 85°C; open squares: 94°C )
References:
Spillaert, R. Hreggvidsson, G.O., Kristjansson, J.K., Eggertsson, G.and Palsdottir, 1994. Cloning and sequencing of aRhodothermus marinus gene, bglA, coding for a thermostable β-glucanase in Eschericha coli. Eur. J. Biochem. 224:923-930
